A Blank Case Report Template for Medical Records provides a structured format for documenting patient information, clinical findings, diagnosis, treatment plans, and outcomes. It ensures consistency and accuracy in medical documentation, facilitating efficient communication among healthcare professionals. Using this template enhances record keeping and supports better patient care management.
Standard Blank Patient Case Report Template
The
Standard Blank Patient Case Report Template document serves as a structured framework for healthcare providers to systematically record detailed patient information, including medical history, symptoms, diagnosis, and treatment plans. It ensures consistency and accuracy in documenting clinical data, facilitating better communication among medical professionals and supporting regulatory compliance. This template is essential for maintaining comprehensive patient records and streamlining clinical trial reporting processes.
Basic Clinical Case Report Form
A
Basic Clinical Case Report Form (CRF) document is a standardized tool used to systematically collect and record data from each participant in a clinical trial. It ensures accurate and consistent data entry regarding patient demographics, medical history, treatment details, and outcome measures. The CRF facilitates data validation and supports regulatory compliance, playing a critical role in the integrity and reliability of clinical research data.
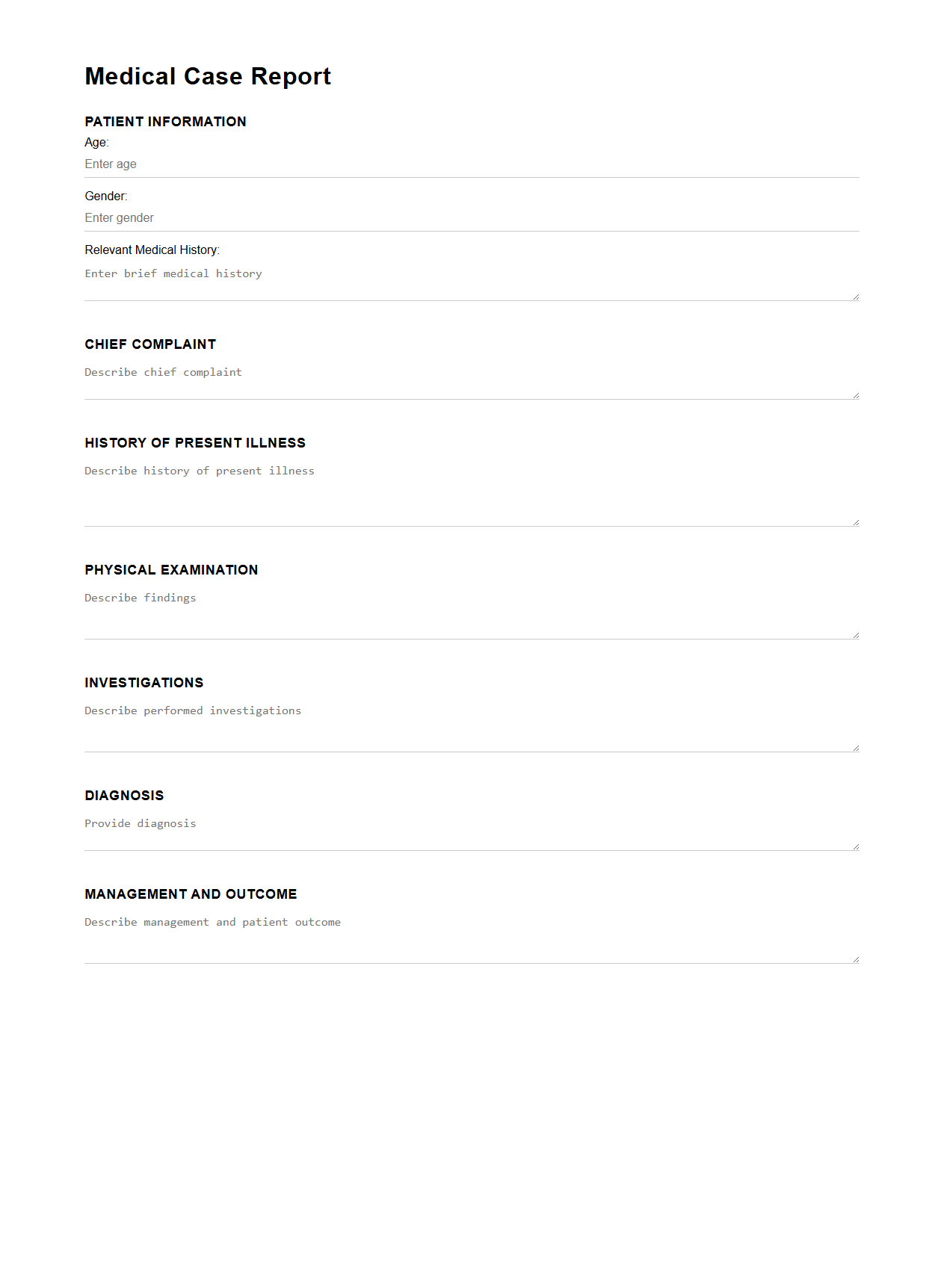
Simple Medical Case Report Outline
A
Simple Medical Case Report Outline document provides a structured framework for organizing clinical case information clearly and concisely. It typically includes sections such as patient history, clinical findings, diagnosis, treatment, and follow-up, ensuring essential details are systematically presented. This format enhances readability, supports accurate medical documentation, and facilitates effective communication among healthcare professionals.
Detailed Patient Case Documentation Sheet
A
Detailed Patient Case Documentation Sheet is a comprehensive medical record that systematically captures all pertinent patient information, including medical history, symptoms, diagnostic results, treatments, and follow-up plans. This document ensures accurate communication among healthcare providers, supports clinical decision-making, and enhances continuity of care throughout the patient's treatment journey. Proper utilization of the sheet improves patient outcomes by facilitating thorough and organized case management.
Initial Medical Case Assessment Template
The
Initial Medical Case Assessment Template document serves as a standardized tool to systematically collect and evaluate a patient's medical history, symptoms, and preliminary examination findings. It helps healthcare professionals organize essential clinical data efficiently, ensuring consistent and thorough case reviews. This template supports accurate diagnosis, treatment planning, and facilitates effective communication across multidisciplinary medical teams.
Structured Case Report for Healthcare Records
A
Structured Case Report for Healthcare Records is a standardized document format designed to systematically capture patient information, clinical findings, diagnoses, treatments, and outcomes. This format enhances data consistency, facilitates interoperability across health information systems, and supports advanced analytics for clinical research and quality improvement. By organizing medical data into predefined fields, it enables efficient data retrieval and improves communication among healthcare providers.
Brief Patient Encounter Report Format
The
Brief Patient Encounter Report Format is a structured document used by healthcare professionals to summarize critical information from a patient's visit efficiently. It typically includes sections for patient identification, presenting complaint, clinical findings, diagnosis, treatment plan, and follow-up instructions. This format ensures clear communication among medical staff and supports accurate record-keeping for ongoing patient care.
Case Summary Documentation Template
A
Case Summary Documentation Template is a structured tool designed to capture and organize essential information about a legal or medical case for quick reference. It includes key elements such as client details, case history, relevant dates, and summaries of findings or actions taken. This template enhances consistency, improves communication among professionals, and streamlines case management processes.
Comprehensive Clinical Report Layout
A
Comprehensive Clinical Report Layout document is a structured template designed to organize and present detailed patient information, diagnostic findings, treatment plans, and clinical observations in a clear and systematic manner. It ensures consistency and completeness in medical reporting, facilitating effective communication among healthcare providers. This layout typically includes sections such as patient history, examination results, laboratory data, imaging studies, and clinical recommendations.
Minimalist Medical Case Writing Template
The
Minimalist Medical Case Writing Template is a streamlined document designed to optimize clarity and efficiency in presenting patient cases. It focuses on essential medical information such as patient history, diagnosis, treatment, and outcomes, while eliminating unnecessary jargon and complex formatting. This template aids healthcare professionals in creating concise, standardized reports that enhance communication and facilitate quicker decision-making.
What patient identifiers should be excluded from a blank case report template to ensure HIPAA compliance?
To ensure HIPAA compliance, patient identifiers such as names, social security numbers, and exact dates related to the individual should be excluded from the blank case report template. Other identifiers like phone numbers, email addresses, and geographic subdivisions smaller than a state must also be omitted. Removing these protects patient privacy while allowing necessary clinical data to be documented.
Which clinical data fields are essential in a blank case report for a rare disease study?
Essential clinical data fields in a blank case report for a rare disease study include demographic details, clinical history, diagnostic criteria, treatment regimens, and outcome measures. Laboratory results and genetic or biomarker data specific to the rare disease should also be captured precisely. These fields ensure comprehensive data collection that supports robust analysis and research.
How should a blank case report letter address consent documentation for retrospective chart reviews?
A blank case report letter should clearly state the requirement for consent documentation or provide an institutional review board (IRB) exemption statement for retrospective chart reviews. It must outline the process for obtaining patient consent or document the waiver, emphasizing compliance with ethical and legal standards. This ensures transparency and protects both patients and researchers.
What standard terminology should be used in a blank case report to align with ICD-10 coding?
To align with ICD-10 coding, the case report must use standardized medical terminology and diagnosis codes consistent with the ICD-10 classification system. Utilizing official code descriptors and ensuring accurate translation of clinical terms facilitate interoperability and data comparability. This approach supports seamless integration into electronic health records and epidemiological studies.
How can a blank case report letter template facilitate multicenter medical records collection?
A blank case report letter template can facilitate multicenter medical records collection by standardizing data fields, instructions, and consent language across all participating sites. It promotes uniformity in data submission, eases coordination, and reduces errors in multicenter studies. Such templates improve data quality and comparability for pooled analyses and collaborative research.